Periostin Shows Promise to Help Fight a Common Form of Esophageal Cancer
Philadelphia | April 9, 2024
Periostin may be a promising novel therapeutic target for treating esophageal squamous cell carcinoma, reports The American Journal of Pathology
Esophageal squamous cell carcinoma (ESCC) accounts for around 90% of esophageal cancers, especially in East Asia. New findings opens in new tab/window in The American Journal of Pathology opens in new tab/window, published by Elsevier, indicate that periostin, or POSTN, promotes ESCC progression by enhancing cancer and stromal cell migration in cancer-associated fibroblasts (CAFs). Therefore, it may be a novel therapeutic target for treating ESCC.
Lead investigator Yu-ichiro Koma, MD, PhD, Division of Pathology, Department of Pathology, Kobe University Graduate School of Medicine, explained, “Though CAFs in the tumor microenvironment are involved in the progression of various cancers, including ESCC, the underlying mechanisms are unclear. Therefore, it is critical to further investigate the mechanisms of esophageal cancer development and progression.”
To better understand the mechanisms of ESCC progression by CAFs, investigators generated CAF-like cells by directly coculturing human bone marrow–derived mesenchymal stem cells (MSCs) with ESCC cells, revealing elevated periostin expression. Recombinant human periostin activated the serine/threonine protein kinase (Akt) and extracellular signal-regulated kinase (Erk) signaling pathways in ESCC cells, which enhanced the survival and migration of these cancer cells. Periostin also enhanced MSC and macrophage migration and conferred tumor-associated macrophage (TAM)-like properties to macrophages. Immunohistochemistry demonstrated the clinical significance of periostin, associating its high expression with tumor invasiveness, vessel invasion, advanced pathological stage, CAF marker expression, and TAM infiltration. After direct coculture, ESCC cells showed increased characteristics of malignancy, such as tumor survival, growth, and migration, as well as increased phosphorylation of Akt and Erk.
Dr. Koma commented: “We discovered that periostin, up-regulated in CAFs upon direct contact with cancer cells, promotes ESCC progression and the migration of stromal cells like CAFs and TAMs. Periostin also enhanced mesenchymal stem cell and macrophage migration and endowed macrophages with tumor-associated macrophage-like properties. Thus, CAF-secreted periostin contributed to tumor microenvironment development.”
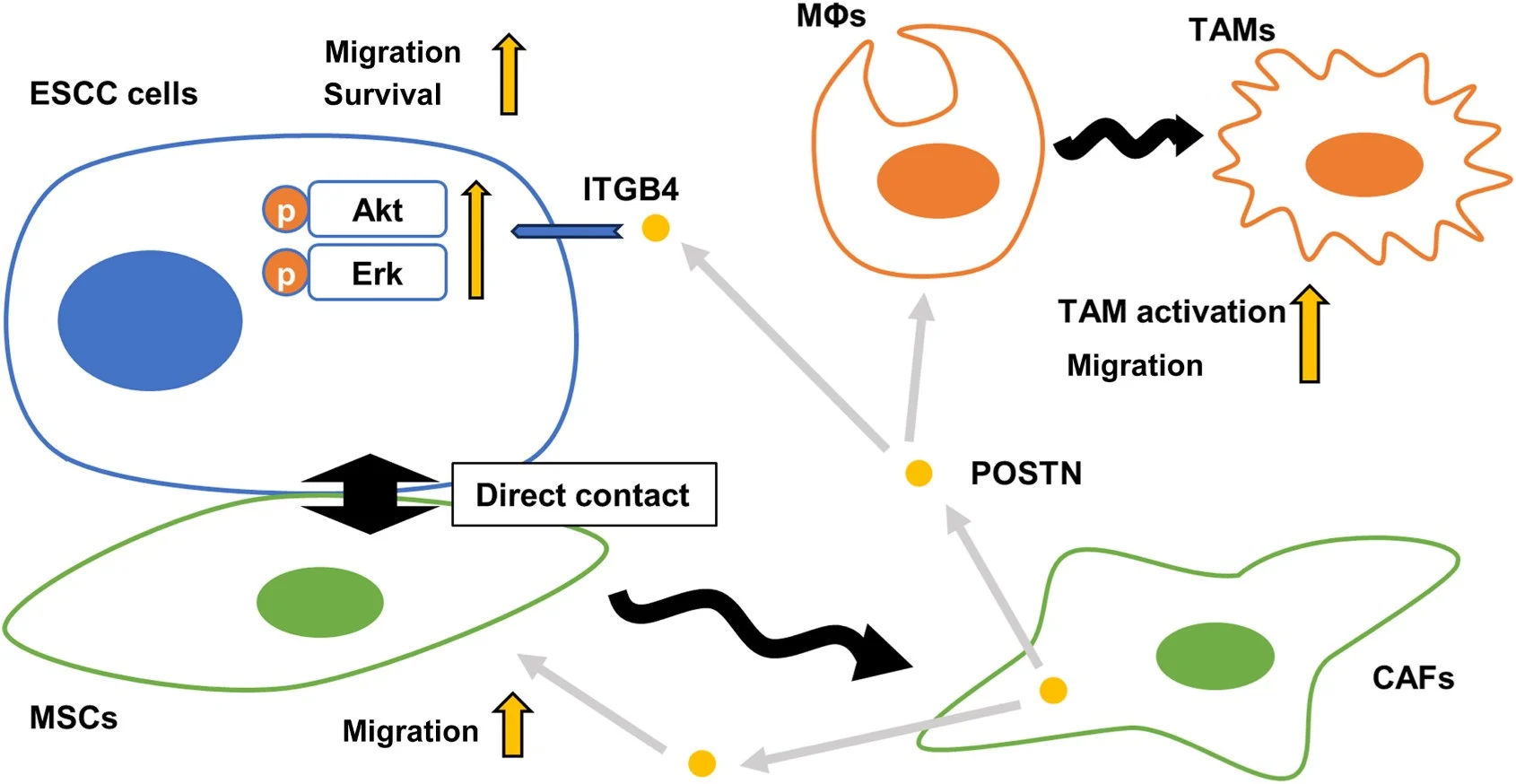
Caption: Schematic diagram of roles of periostin (POSTN) in the esophageal squamous cell carcinoma (ESCC) microenvironment. Direct contact with ESCC cells leads mesenchymal stem cells (MSCs) to become cancer-associated fibroblast (CAF)-like cells, which secrete periostin and activate the Akt and extracellular signal-regulated kinase (Erk) pathways via integrin subunit beta 4 (ITGB4) in ESCC cells, promoting their migration. Periostin also promotes MSC and macrophage migration and contributes to the activation of tumor-associated macrophage (TAM)-like macrophage properties. MΦ, macrophage (Credit: The American Journal of Pathology).
Dr. Koma concluded: “The present study established a direct coculture system between ESCC cells and MSCs, which promoted the malignant phenotype of ESCC cells. Patients with ESCC with high periostin expression exhibited poorer postoperative outcomes, indicating that periostin may be a novel therapeutic target for treating this form of esophageal cancer and may serve as a prognostic indicator.”
Esophageal cancer is the seventh most common cancer worldwide and the sixth leading cause of cancer-related death. The most common histological subtype is ESCC. The histologic types of esophageal cancer are broadly classified into ESCC and esophageal adenocarcinoma, with ESCC accounting for approximately 90% of esophageal cancers, especially in East Asia. In East Asia and East Africa, esophageal cancer is one of the top five causes of overall cancer mortality, and the five-year overall survival of ESCC is approximately 20%, with a poor prognosis. This prognosis is associated with a high propensity for metastasis, even if the tumor is superficial, Early esophageal cancer is often asymptomatic and diagnosis typically occurs at an advanced stage.
Notes for editors
The article is “Periostin in Cancer-Associated Fibroblasts Promotes Esophageal Squamous Cell Carcinoma Progression by Enhancing Cancer and Stromal Cell Migration,” by Shoji Miyako, Yu-ichiro Koma, Takashi Nakanishi, Shuichi Tsukamoto, Keitaro Yamanaka, Nobuaki Ishihara, Yuki Azumi, Satoshi Urakami, Masaki Shimizu, Takayuki Kodama, Mari Nishio, Manabu Shigeoka, Yoshihiro Kakeji, and Hiroshi Yokozaki (https://doi.org/10.1016/j.ajpath.2023.12.010 opens in new tab/window). It appears online ahead of The American Journal of Pathology, volume 194, issue 5 (May 2024), published by Elsevier.
The article is openly available athttps://ajp.amjpathol.org/article/S0002-9440(24)00040-3/fulltext opens in new tab/window.
Full text of the article is also available to credentialed journalists upon request. Contact Eileen Leahy at +1 732 406 1313 or [email protected] opens in new tab/window to request a PDF of the article or more information or to reach the authors for comment.
This study was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science and Takeda Science Foundation.
About The American Journal of Pathology
The American Journal of Pathology opens in new tab/window, official journal of the American Society for Investigative Pathology opens in new tab/window, published by Elsevier, seeks high-quality original research reports, reviews, and commentaries related to the molecular and cellular basis of disease. The editors will consider basic, translational, and clinical investigations that directly address mechanisms of pathogenesis or provide a foundation for future mechanistic inquiries. Examples of such foundational investigations include data mining, identification of biomarkers, molecular pathology, and discovery research. High priority is given to studies of human disease and relevant experimental models using molecular, cellular, and organismal approaches. https://ajp.amjpathol.org opens in new tab/window
About Elsevier
A global leader in advanced information and decision support, Elsevier helps to advance science and healthcare, to advance human progress. We do this by facilitating insights and critical decision-making with innovative solutions based on trusted, evidence-based content and advanced AI-enabled digital technologies.
We have supported the work of our research and healthcare communities for more than 140 years. Our 9,700 employees around the world, including 2,300 technologists, are dedicated to supporting researchers, librarians, academic leaders, funders, governments, R&D-intensive companies, doctors, nurses, future healthcare professionals and educators in their critical work. Our 3,000 scientific journals and iconic reference books include the foremost titles in their fields, including Cell Press, The Lancet and Gray’s Anatomy. Together with the Elsevier Foundation opens in new tab/window, we work in partnership with the communities we serve to advance inclusion in science, research and healthcare in developing countries and around the world.
Elsevier is part of RELX opens in new tab/window, a global provider of information-based analytics and decision tools for professional and business customers. For more information on our work, digital solutions and content, visit www.elsevier.com.
Contact
EL
CCP
Chhavi Chauhan, PhD
Director of Scientific Outreach
The American Journal of Pathology
+1 240 283 9724
E-mail Chhavi Chauhan, PhD