Addressing the process safety concerns of hydrogen in a net zero economy
2022년 10월 17일
저자: Starr Tze, P.Eng., Kathryn Grant, Erik Sherwin

A 3D rendering of a hydrogen pipeline (© istock.com/petmal)
While “green hydrogen” is a viable fuel option, production can be risky — Process Safety Board members explain how to reduce the risk
Hydrogen is seen by many as a potential option for reducing the use of hydrocarbons and as a viable fuel source. It has applications in the three sectors representing some of the toughest hurdles in the race toward net zero: power generation, oil & gas, and transportation.
However, one of the first challenges to address is how that hydrogen is produced. The demand for pure hydrogen has grown by more than 100% since 1975, yet 95% is made from natural gas and coal through steam reforming, according to the US Office of Energy Efficiency & Renewable Energy 새 탭/창에서 열기. This type of hydrogen is not considered sustainable over the long term. It’s currently the cheapest forms of hydrogen, however, and is likely to play a bridging role in the emerging hydrogen economy. Technologies such as carbon capture, utilization and storage (CCUS) can be used to reduce the greenhouse gases and other harmful emissions released as part of traditional hydrogen production processes.
On the other hand, hydrogen that is produced through electrolysis using renewable energy is regarded as green hydrogen. It is viewed as an attractive fuel option for transportation and electrical generation applications in addition to oil refining, ammonia production, methanol production and steel production.
That’s why the transition from traditional methods of hydrogen production to renewable sources is being strongly supported by policies in various countries. These encourage investment into renewable hydrogen production to bring the technology to maturity and reduce costs to enable it to be competitive with carbon-based production methods. Currently, green hydrogen is up to eight times more expensive compared to electricity sourced from coal.
Electrolysis takes place within an electrolyzer that uses electricity to split water into hydrogen and oxygen. There are several different types electrolyzers, but all consist of three primary components: an anode, a cathode and an electrolyte to separate the anode and cathode. The main byproduct of electrolysis is oxygen, which can be used for other applications.
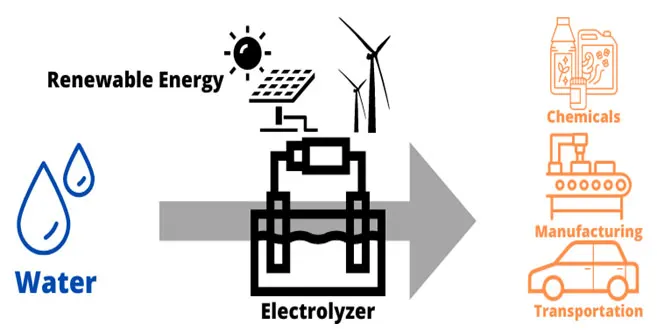
Figure 1: Sustainable green hydrogen electrolysis process. (Image © Process Safety Board)
Mitigating the hazards of hydrogen
A big advantage of hydrogen is its high energy content per mass. Yet this poses a significant safety hazard. Hydrogen has a wide flammability range (44.0-75 vol%) and a low ignition energy (0.018 mJ) compared to other fuel sources such as methane (5.3-15 vol% flammability, 0.280 mJ ignition energy) and gasoline (1.0-7.6 vol% flammability, 0.25 mJ ignition energy). A small leak from piping or a storage vessel could easily be ignited, leading to a fire or explosion.
Further factors to consider include the low viscosity and low density of hydrogen. It can also diffuse through certain materials faster than other gasses, again posing risk of leaks and loss of containment. According to studies, hydrogen can leak from a system about three times faster than natural gas on a volumetric basis. Therefore, proper handling is essential all the way through the value chain, including production, storage, transportation (via pipelines, truck or rail) and final usage.
Several damage mechanisms must also be noted specific to hydrogen. Embrittlement of metals and other materials can result in loss of integrity and eventual loss of containment. An example of this type of failure occurred at the Tesoro Refinery in Anacortes, Washington, in 2010. A heat exchanger containing hydrogen and naphtha ruptured due to high-temperature hydrogen attacking the piping. Embrittlement eventually led to an explosion that killed seven employees — the largest number of fatalities in an incident at a US petroleum refinery in many years.
To mitigate embrittlement, the use stainless steel or aluminum are generally recommended for most processes. Process safety engineers should apply the principle of inherently safer design and select the most appropriate materials for each application.
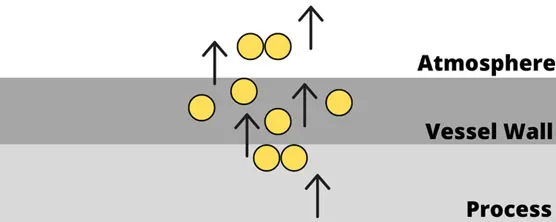
Figure 2: High-temperature hydrogen attack of atomic hydrogen diffusing through steel vessel wall. (Image © Process Safety Board)
Another risk to consider is decarburization — the decrease of the carbon content of metals such as steel when heated to temperatures of 700 °C or above. As a result, the carbon present in the metal reacts with oxygen and hydrogen and leads to softening. In hydrogen processes, free carbon can bond with the hydrogen and create pockets of methane. This again poses a safety risk.
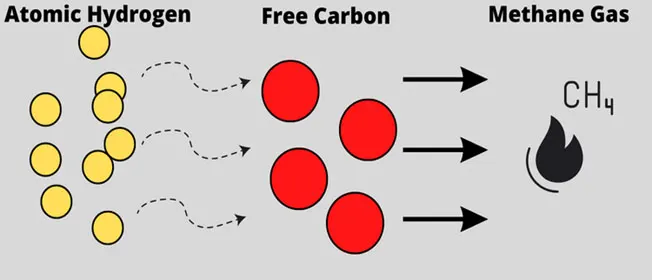
Figure 3: Decarburization when atomic hydrogen bonds with free carbon in steel and creates pockets of methane gas (Image © Process Safety Board)
Process safety engineers help reduce the likelihood of these types of incidents. Every injury, fatality or incidence of infrastructure damage can negatively impact public acceptance of hydrogen and ultimately slow the energy transition. Therefore, process safety is a critical part of the path to net zero emissions and the development of new energy sources.
Resources
For more information on this topic or other trends in process engineering and engineering safety, the Process Safety Board 새 탭/창에서 열기 offers learning opportunities including videos, articles and live events, as well as access to a community of process safety experts.
Visit the Process Safety Board website 새 탭/창에서 열기
Elsevier’s Knovel contains a wealth of knowledge related to hydrogen. This includes research and recommendations concerning the selection of appropriate metals and other materials, how to avoid or mitigate corrosion, and how to enhance process safety. It helps engineers improve asset integrity and plant efficiency, reduce downtime, conduct failure analysis, lower operational risk, and discover environmentally friendly approaches to the deployment of green hydrogen solutions.
기여자

STP
Starr Tze, P.Eng.

KG
Kathryn Grant

ES