Quick Facts
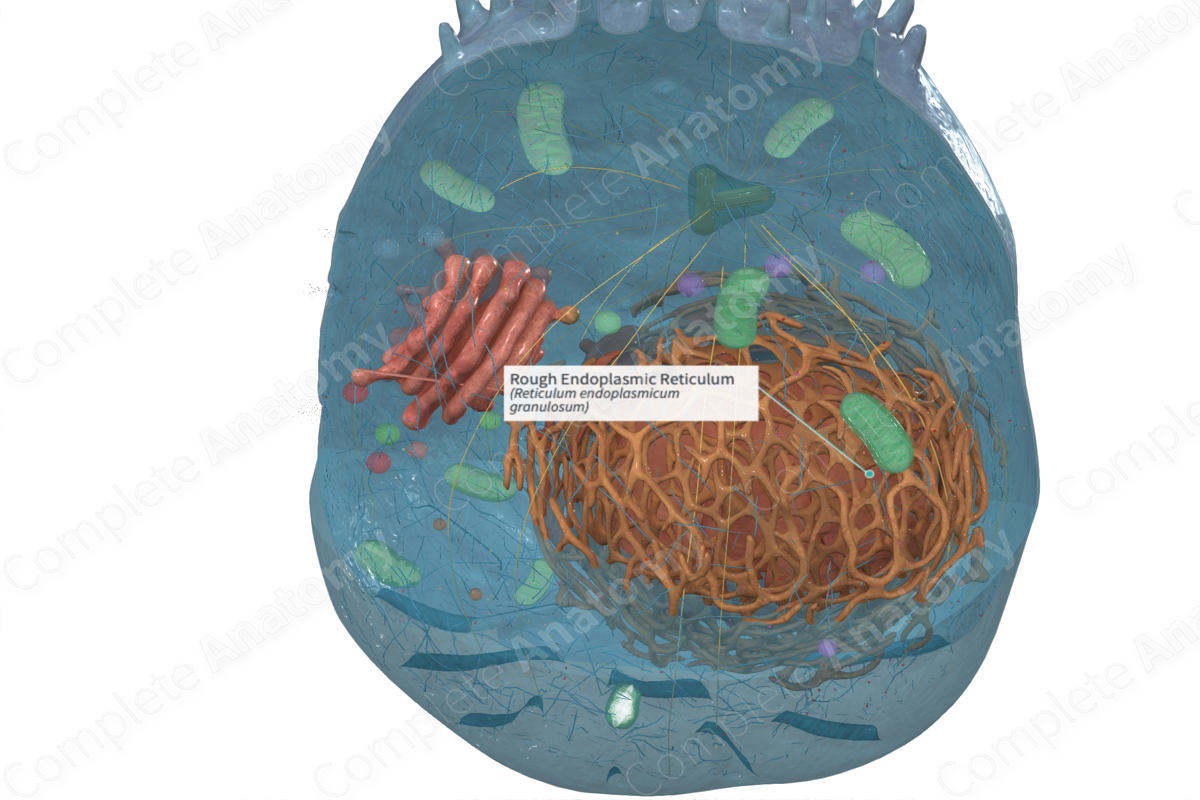
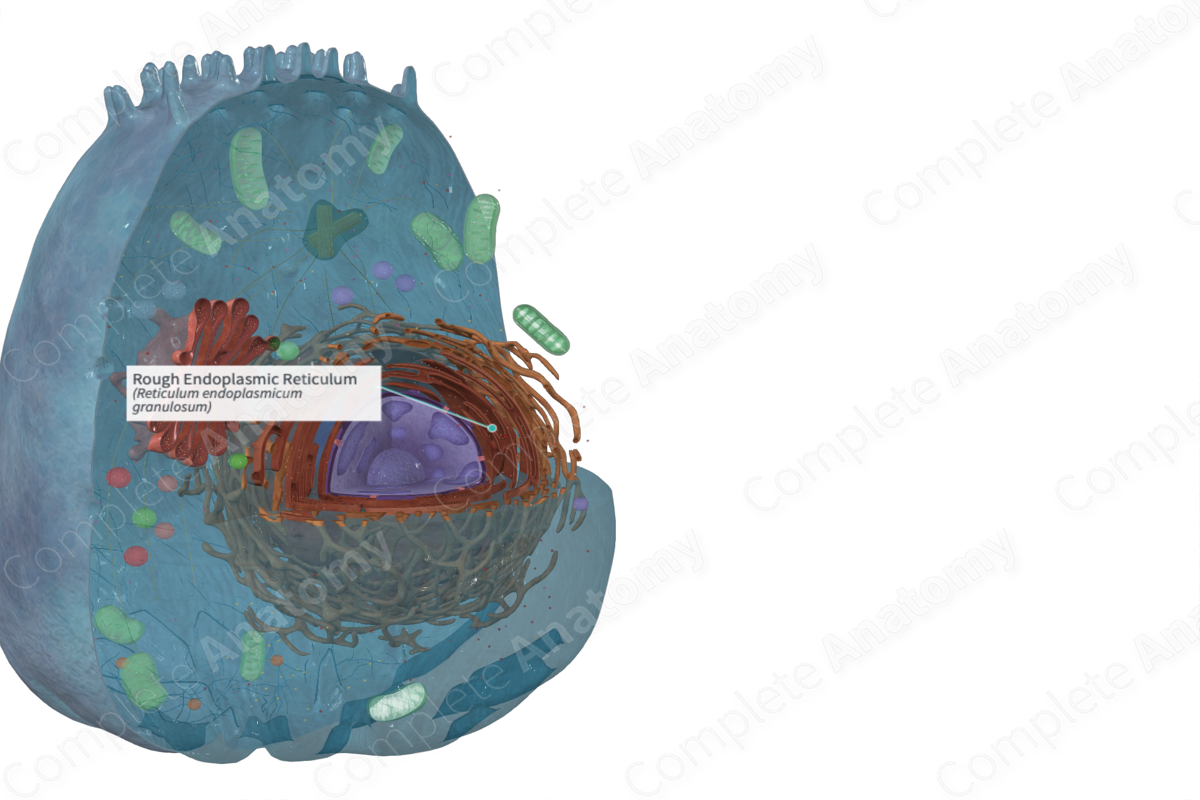
The rough endoplasmic reticulum is a region of the endoplasmic reticulum that bears large numbers of ribosomes on the outer surface of its membrane and is specialized for protein synthesis and folding (Dorland, 2011).
Structure and/or Key Feature(s)
The endoplasmic reticulum is a vast membranous network throughout the cell and is the largest organelle in the cell. It consists of sacs, cisterns (or passageways) interconnecting tubules and comes in two forms that each can extend the full extent of the cell cytoplasm.
One form is called rough endoplasmic reticulum (rER). It’s a network of flattened membranous sacs termed cisternae. It’s also known as granular endoplasmic reticulum (gER), because it has ribosomes and/or polyribosomes associated with the cytosolic side of the membrane. These ribosomes attached to the external face of the membranes of the rough endoplasmic reticulum are attached only when a protein is being made. They become detached after the protein is completed (Ross and Pawlina, 2006; Ovalle, Nahirney and Netter, 2013; McKinley, O'Loughlin and Pennefather-O'Brien, 2016).
Anatomical Relations
The rough endoplasmic reticulum (rER) is continuous with the membrane of the nuclear envelope.
Function
The rough endoplasmic reticulum possesses enzymes and protein complexes. Its protein complexes function as molecular chaperones which guide the nascent (newly formed) protein folding, inhibit aggregation, and monitor the quality of the proteins within the rough endoplasmic reticulum.
Proteins that are synthesized within the rough endoplasmic reticulum are either destined to become:
- intracellularly stored, such as in lysosomes and specific granules of leukocytes;
- intracellularly stored prior to exocytosis, such as in exocrine cells;
- incorporated as integral membrane proteins into the plasmalemma.
The rough endoplasmic reticulum also exhibits a mechanism that prevents faulty proteins that did not fold or assemble properly from being forwarded to other organelles or for secretion. This mechanism is known as the endoplasmic reticulum associated degradation. With this mechanism, unsalvageable proteins translocate back into the cytosol, become conjugated with a regulatory protein known as ubiquitin and are eventually degraded by proteasomes (Mescher, 2013).
The region of the cytoplasm actively synthesizing proteins stains intensely with basic dyes due to the presence of RNA. Large clumps of rough endoplasmic reticulum in large motor neurons are called Nissl bodies or Nissl substance (Ross and Pawlina, 2006).
Clinical Correlates
If the endoplasmic reticulum is stressed, it may activate the “unfolded protein response” (UPR) due to abnormal proteins accumulating within the cistern of the endoplasmic reticulum. The UPR attempts to restore normal function by the endoplasmic reticulum but dysfunction of the UPR can lead to a number of neurodegenerative diseases (Ovalle, Nahirney and Netter, 2013).
References
Dorland, W. (2011) Dorland's Illustrated Medical Dictionary. 32nd edn. Philadelphia, USA: Elsevier Saunders.
McKinley, M. P., O'Loughlin, V. D. and Pennefather-O'Brien, E. E. (2016) Human Anatomy. 5th edn.: McGraw-Hill Education.
Mescher, A. (2013) Junqueira's Basic Histology: Text and Atlas. 13th edn.: McGraw-Hill Education.
Ovalle, W. K., Nahirney, P. C. and Netter, F. H. (2013) Netter's Essential Histology. ClinicalKey 2012: Elsevier Saunders.
Ross, M. H. and Pawlina, W. (2006) Histology: A text and atlas. Lippincott Williams & Wilkins.