The most powerful way to advance your drug portfolio | PharmaPendium. Find it. Predict it.

Lamentablemente no somos totalmente compatibles con su navegador. Si tiene la opción, actualice a una versión más reciente o utilice Mozilla Firefox, Microsoft Edge, Google Chrome o Safari 14 o posterior. Si no puede y necesita ayuda, envíenos sus comentarios.
Agradeceríamos sus comentarios sobre esta nueva experiencia.Díganos qué piensa se abre en una nueva pestaña/ventana
The translation of research findings to humans is one of the greatest challenges. Improve patient safety and assess the benefit-risk ratio with PharmaPendium's calculation tools for predictive analysis: - Drug-Drug Interaction Risk Calculator (DDIRC) - Safety Margin Tool
Purposeful prediction, powerful translation.

The Drug-Drug Interaction Risk Calculator (DDIRC) was developed in collaboration with Sanofi, Merck, Servier, Boerhinger Ingelheim and others with the aim of improving patient safety. The tool is part of PharmaPendium's DMPK (Drug Metabolism and Pharmacokinetics) solution.
The DDIRC helps you predict harmful drug-drug interactions (DDI) fast and as early as possible in drug development. Calculate the area under the curve (AUC) changes to optimize clinical trial design and avoid unnecessary DDI trials. An entire arm of a clinical study and time may be saved. The DDIRC supports your decision making to fail weak candidates fast and select the most promising.
This mechanistic static tool includes perpetrators and victims along with their in vitro and in vivo data — the most comprehensive dataset in a single solution. The DDIRC is compliant with the 2020 FDA guidelines, as well as with EMA and PMDA.
"Because patient safety has always been a priority for Servier, we want to make sure that our drugs are optimally co-administered. Predicting pharmacokinetic Drug-Drug Interactions (DDI) with the maximum of relevance, precision and reactivity is therefore essential. DDIRC is therefore an essential tool … and enables rapid responses, hence decisions, on the interaction risks."
YP
Yannick Parmentier
Head of the Biopharmaceutical Research Department en Servier
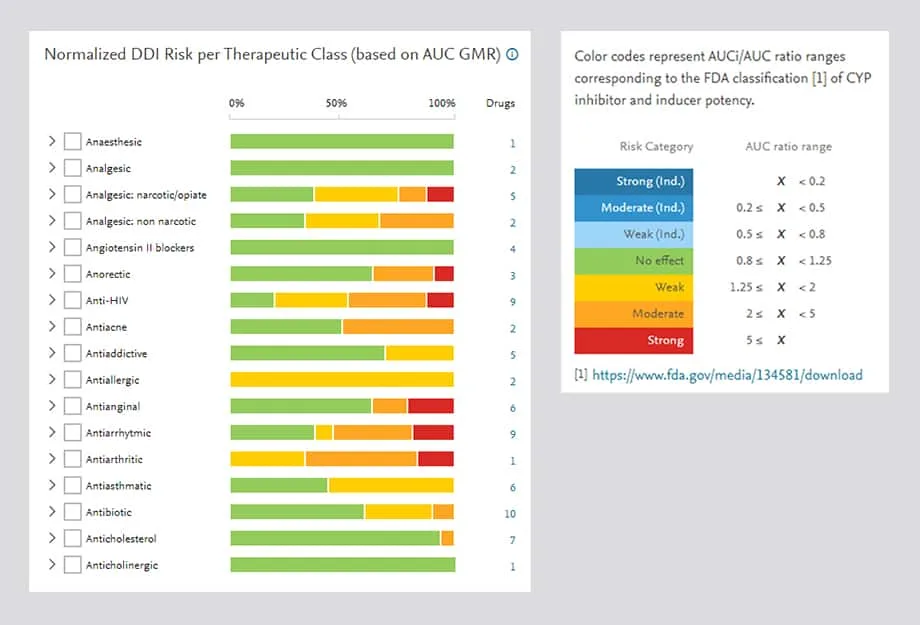
The DDIRC is powerful and easy to use. Advanced visualization and filters support the quick identification of drug-drug interaction risk. In the visualization of risk per therapeutic classes, the colors indicate a spectrum from no risk to high risk.

Sanofi/DNDi use PharmaPendium's Drug-Drug Interaction Risk Calculator in successful EMA and FDA submissions
Learn how DDIRC predictions supported the authorization of fexinidazole. se abre en una nueva pestaña/ventanaPharmaPendium’s new Safety Margin Tool supports you in terms of secondary pharmacology. It can help you predict the risk of off-target adverse drug reactions (ADRs) for your drug candidates, answering questions such as:
What dose of a small molecule is likely to be safe for use in humans?
Which drug candidate is likely to be the safest?
The Safety Margin Tool was developed in a longstanding collaboration with Novartis in response to biopharma organizations’ needs.
By combining target affinity data from literature and PK/PD data from marketed and withdrawn drugs, a safety margin is calculated. This tool will help you predict which drug candidates need be failed as early as possible because of high probability of off-target ADRs and which ones will likely become successful additions to your drug portfolio.
"Of utmost importance is PharmaPendium's role in providing information about clinical adverse events, allowing for the identification of underlying toxicity in nonclinical studies."

GB
Guy Bouvier, PhD, ERT
Director, Toxicology & Product Safety en Groupe Pierre Fabre
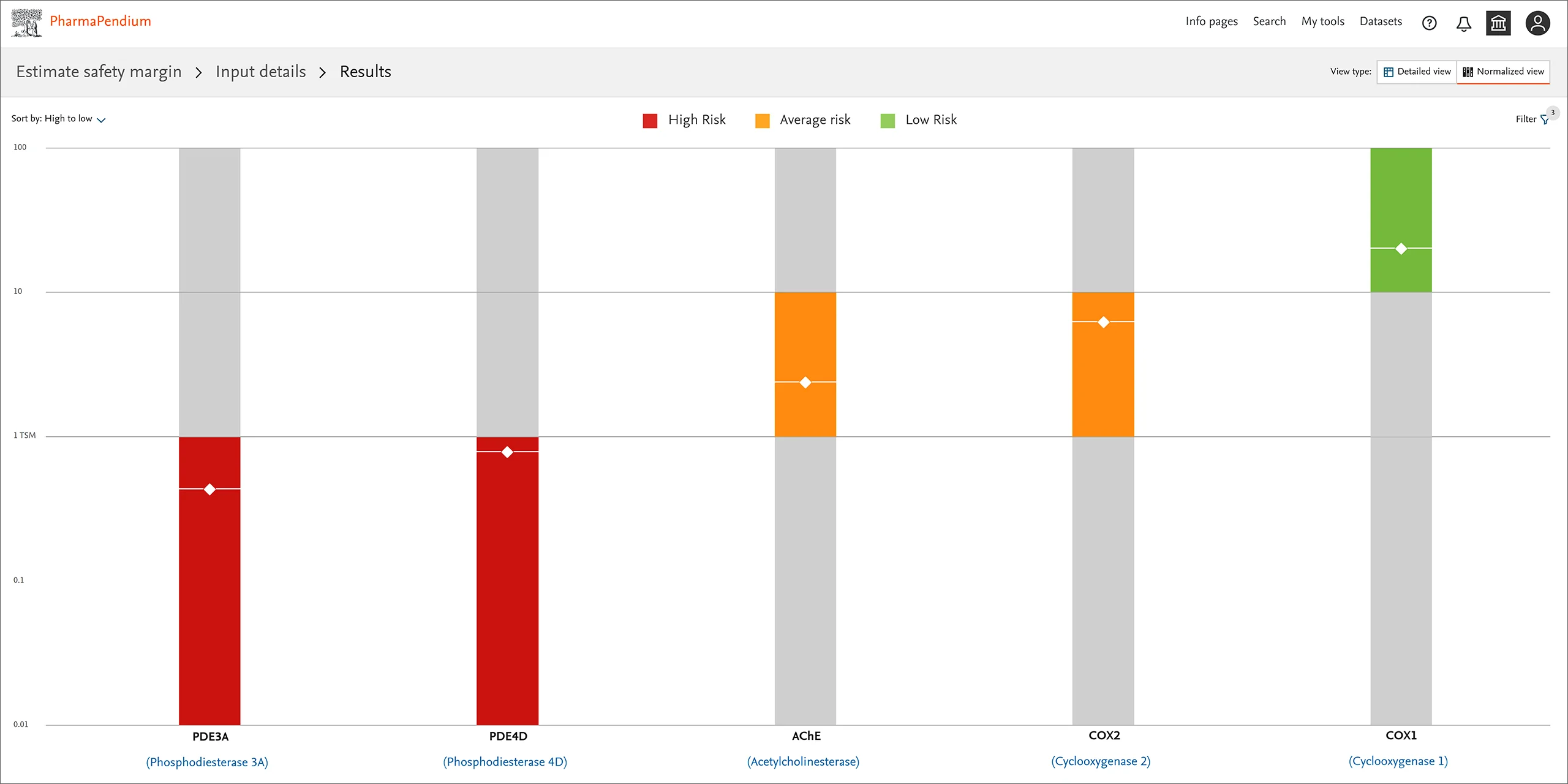
Safety Margin Tool normalized view - results page: Identify and address safety hazards within the early phase of drug discovery by semi-quantitative off-target mitigation.
