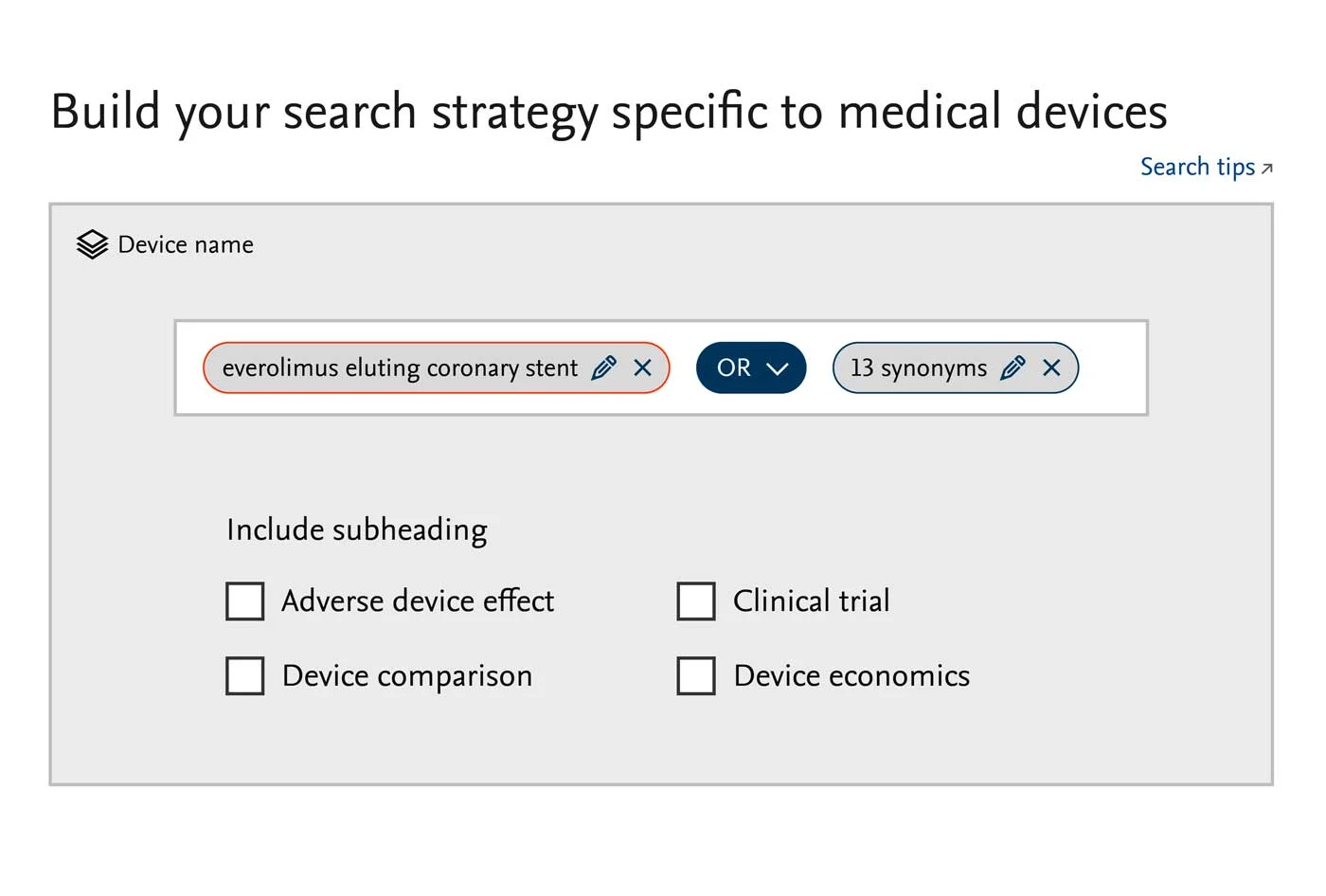
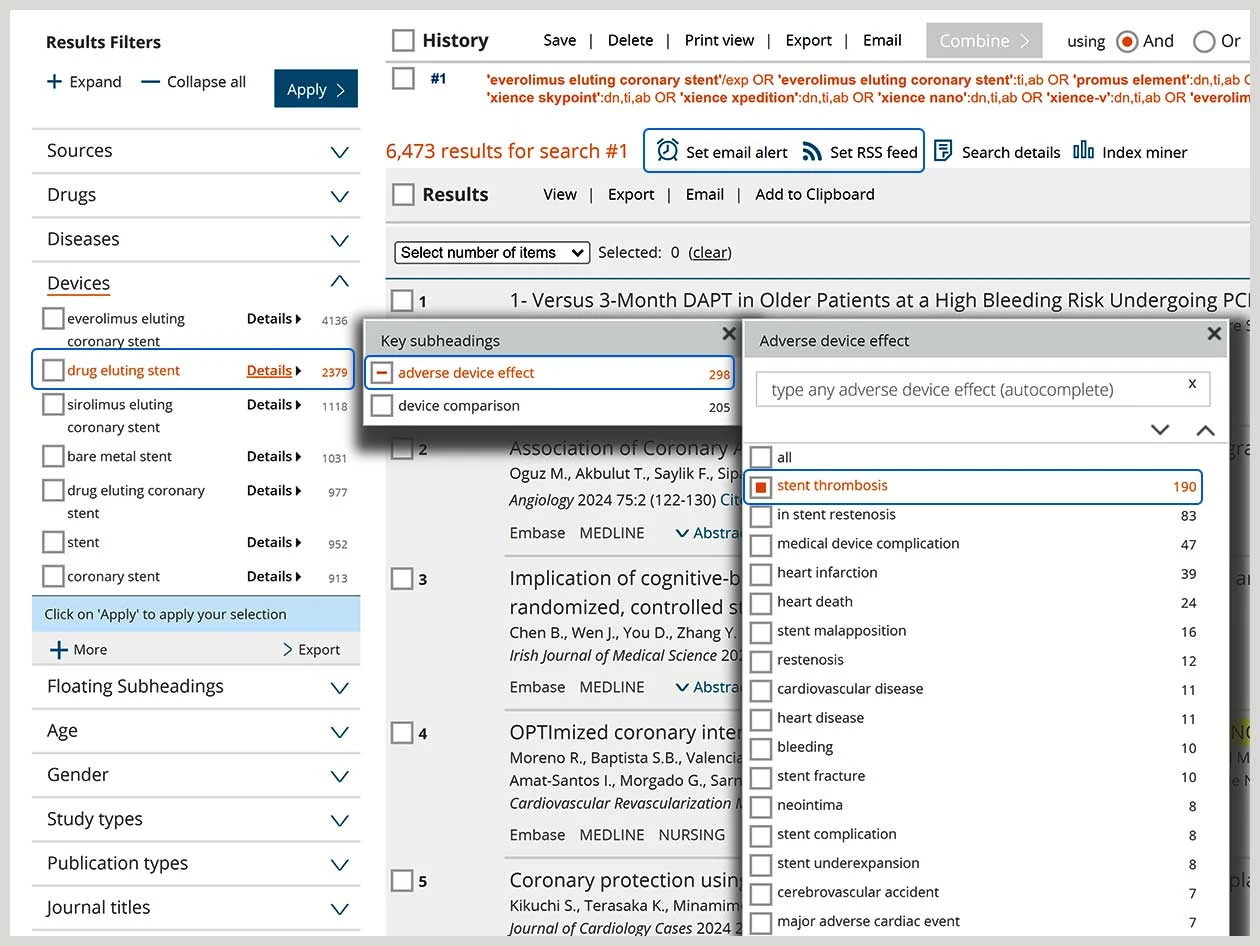
Support for the medical device life cycle
Embase has the medical device research, evidence and literature monitoring tools to help you:
Develop and validate new concepts
Comply with medical device regulations
Prepare clinical evaluation reports for CE Marketing approval
Conduct post-market surveillance